Make a change today!
"Real Time" Magazine is an online, self-funded news magazine, founded with a firm idealogy to make a different, alternative, uncensored quality news, accessible and direct to and with the public.
Our team members currently work as volunteers and we would DEEPLY appreciate and love your support, so we can keep provide what is direly missing in mainstream media.
click for single / monthly support
Thank you ??
Anatomy of concealment: Skeletal deformities and double abortion rate in Pfizer's animal studies; In women, almost all pregnancies in Pfizer's April 2021 report with known outcome ended in fetal death
Documents obtained through FOI Act in the US and Australia reveal: The FDA ignored the serious findings and did not require the company to perform further toxicity tests. The findings were concealed and the vaccine was classified as safe for use in pregnancy


Of all the side effects associated with COVID-19 vaccines, the most silenced are those associated with miscarriage and stillbirths. Any attempt to talk about the issue is ridiculed, suppressed and censored by the authorities and the
media, who claim that there is no scientific evidence that these vaccines can harm pregnancy or fetuses. Furthermore, it was claimed that animal studies conducted with the Pfizer, Moderna and Janssen vaccines have not found any teratogenic or fetotoxic effects for any of these vaccines.
However, it now appears that health authorities have withheld critical information from the public: Animal toxicity studies conducted by Pfizer to test the teratogenicity of the vaccine in pregnancy have indicated serious problems, which were manifested both in a high rate of miscarriages and in skeletal development in surviving fetuses. Nevertheless, the regulators did not require the company to conduct further experiments that would clarify the degree of risk, and even concealed the problematic findings and changed the safety classification of the vaccine in pregnancy, from a classification according to which the risk is unknown, to a classification that states that the vaccine is safe in pregnancy.
At the same time, Pfizer's adverse-effects report submitted to the FDA in April 2021, which was revealed through a Freedom of Information Claim filed in the United States, also showed alarming findings regarding pregnant women vaccinated during the first two and a half months followinf the launch of the vaccination campaign - almost all pregnancies for which the results were known to Pfizer at the time of filing the report ended in fetal death.
The serious findings were concealed by the regulators and the classification was deleted.
The findings regarding the serious problems in Pfizer's animal study were revealed following a freedom of information (FOI) request to the Australian Medicines Agency. It all started when a phycisian, whose Identity and personal information remained confidential, submitted an application under the FOI to the Australian Authority in February 2021, requesting, among other things, the animal study report examining the toxicity of the vaccine and its impact on fertility and reproduction - a study that the authority stated was carried out by the company, but without providing a source that could be read and evaluated independently. On May 21, 2021, after repeated correspondence with the physician who submitted the request, the Australian authority finally published the study report (TGA FOI 2289).
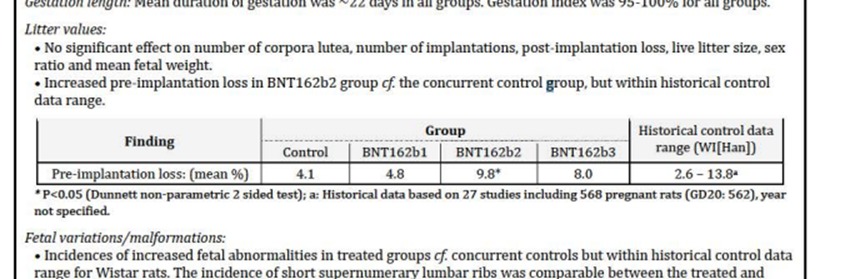
The report shows that the study findings ostensibly indicate that no adverse effect was found on ejaculation cycles, mating, fertility or any parameter of the ovaries or uterus in female rats injected with 4 doses - 2 before fertilization and 2 during pregnancy, and not on pregnancy or calving, including fetal development, internal organs and skeleton, or functional development of puppies.
However, it turns out that there were several previous versions of the document, which show that the rat study indicated serious problems, and that regulators were aware of this and even initially classified it as an unknown risk of pregnancy - but the serious findings were concealed by regulators, and the classification was deleted, and in its place a new classification was established, according to which the vaccine is safe for use in pregnancy. The documents were subsequently disclosed, through additional requirements under the FOI Act from the Australian Medicines Agency (TGA FOI 2389).
It turns out that the study report had several previous versions, which show that the rat study indicated serious problems, and that regulators were aware of this and even initially classified it as having an unknown risk of pregnancy - but the serious findings were concealed and the classification was deleted.
One of the exposed documents stated that among the embryos of the rats injected with Pfizers' vaccine during pregnancy, an "Increased incidence of supernumerary lumbar ribs in rat fetuses" was observed (p. 8). This comment was also noted by the Australian Authority on page 27, where the authority asks the company to make changes in Chapter 4.6 - "Fertility, Pregnancy and lactation". In light of this, the regulator informed Pfizer that the pregnancy vaccine was classified as "Pregnancy Category B2" - an unknown risk, and the chapter concludes: "Administration of COMIRNATY in pregnancy should only be considered when the potential benefits outweigh any potential risks for the mother and fetus".
 Yet, another document, which appears to have been written later, suggests that regulators tried to disguise this dire finding, arguing that its relevance to humans is unclear, and classified it as Category B1, a classification that means animal studies showed no risk. On page 8 of the paper it was argued that the Vaccine Advisory Committee “noted that nonclinical studies do not suggest direct or indirect harmful effects with regard to fertility, embryo-fetal development or post-natal development”, and that it is unclear whether reports of extra lumbar ribs in rats are clinically relevant to humans. "Following additional information from the sponsor, the TGA evaluators now agreed with the sponsor’s proposal of Use in Pregnancy Category B1". The document also states that "It was felt there are no significant concerns for the use of this vaccine in breastfeeding women".
Yet, another document, which appears to have been written later, suggests that regulators tried to disguise this dire finding, arguing that its relevance to humans is unclear, and classified it as Category B1, a classification that means animal studies showed no risk. On page 8 of the paper it was argued that the Vaccine Advisory Committee “noted that nonclinical studies do not suggest direct or indirect harmful effects with regard to fertility, embryo-fetal development or post-natal development”, and that it is unclear whether reports of extra lumbar ribs in rats are clinically relevant to humans. "Following additional information from the sponsor, the TGA evaluators now agreed with the sponsor’s proposal of Use in Pregnancy Category B1". The document also states that "It was felt there are no significant concerns for the use of this vaccine in breastfeeding women".
Moreover, in another document, which appears to have been written even later than the previous one, the findings on excess ribs found in the rat embryos were completely removed. Instead it is written briefly (on page 5): "A combined reproductive and developmental study showed no adverse effects on female fertility, embryofetal development and post-natal development (up to weaning) in rats". In light of this, it read: ". Pregnancy category B1 is considered acceptable". On page 15 of the same document, which again includes the regulator's comments to the company regarding Chapter 4.6 - "Fertility, Pregnancy and Lactation", it can be seen that in the heading "Pregnancy Category B2" - the number 2 in classification B2 was deleted, and the number 1 was written instead. Here too it was written "A combined fertility and developmental toxicity study in rats did not show vaccine-related harmful effects on embryofetal developmen". However, it was noted that Administration of COMIRNATY in pregnancy should only be considered when the potential benefits outweigh any potential risks for the mother and fetus".
However, the same document, on page 55, presents a table omitted from previous versions of the report provided by the Australian Authority, which shows that beyond the developmental problem in the fetal skeletion, another serious finding was found in the study - the abortion rate was also high, and actually double comapred to the group that did not recieve the Pfizer vaccine.
And in another document, which includes the final version of Pfizer's Commiranty Vaccine product information leaflet, Chapter 4.6 - "Fertility, Pregnancy and Lactation" is again included (p. 8) - and it can be seen that indeed, the vaccine was indeed finally classified as Category B1.
The animal study phase - the pre-clinical studies, is a critical phase in the approval process of any drug preparation, which pharmaceutical companies are required to complete before they are given the green light to proceed to the clinical studies phase - with human participants. Particularly critical are animal toxicity studies designed to test the safety of new drug preparations in pregnancy and on fetal development. However, as far as the COVID-19 vaccines are concerned, it seems that the authorities have waived Pfizer's completion of the animal studies, authorizing the company to begin clinical trials and even eventually approving the vaccine without these studies being completed.
This fact is clear from the official instructions of the UK Government regarding Pfizer's mRNA vaccine. The guidelines document explicitly states that studies on the toxicity of the vaccine on animal reproduction were not completed when the vaccine received the emergency permit, and that it is not known whether the vaccine has an impact on fertility.

The guidelines document explicitly states that studies on the toxicity of the vaccine on animal reproduction were not completed when the vaccine received the emergency use permit, and that it is not known whether the vaccine has an impact on fertility.
Perhaps this is why in the clinical study conducted by the company for the purpose of obtaining the emergency use authorization, not only was pregnancy one of the exclusion criteria in the study, but women and men of childbearing age were asked to use contraceptives, or even "agree to remain abstinent". According to Section 10.4.2 of the Pfizer / Biontech Study Protocol (p. 132), a woman of childbearing age is eligible to participate if she is not pregnant or breastfeeding, and if she is using contraception as described in the study protocol during the treatment period (for a minimum of 28 days after last dosing). Men seeking to participate in the study had to pledge not to donate sperm or have sex with women of childbearing age to avoid becoming pregnant. This, during all participation in the experiment and at least for 28 days after the second dose, "which corresponds to the time needed to eliminate reproductive safety risk of the study intervention(s)", the document states.

The serious findings were concealed and the risk classification was deleted
The data on miscarriages and stillbirths from Pfizer's clinical study were unveiled as part of a requirement under the FOI Act filed in the US last September by the Public Health and Medical Professionals for Transparency (PHMPT) - An organization of physicians, scientists, and public health experts working in some of the leading institutions in the US.
Four days after the FDA approved the vaccine for ages 16 and older, the organization, through attorney Aaron Siri, applied under the FOI Act to obtain all of the data in the vaccine's biological product file. But the FDA did not respond to the request, and Siri filed a lawsuit on behalf of the organization against the FDA, demanding that the information be disclosed within 108 days - the same amount of time it took the FDA to review Pfizer's data and grant the vaccine an emergency use authorization. The FDA argued in court that it did not have enough manpower to examine the documents during the time required in the lawsuit, and initially sought to release the documents in a slow drip, which would bring them to the disclosure of all documents only in 2076 - in 55 years. Subsequently, the authority claimed that it had found additional documents, that even that time will not be enough, and asked to give it an extension of at least another 20 years, that is, until 2096. Fortunately, the federal court did not accept the claim and ruled on Jan. 12 that the FDA and Pfizer would have to respond to Freedom of Information requests and provide 55,000 documents every month. Currently, the FDA has not appealed the decision.
Among the documents submitted by the FDA , one of the first reports submitted to Pfizer was "Cumulative Analysis of Post-Approval Adverse Event Reports of PF-07302048 (BNT162B2) Received Thorugh 28-Feb-2021", prepared by Pfizer on April 30th. This document describes events reported to Pfizer from December 11 to February 28, 2021 - that is, about two and a half months since the vaccine received the emergency use permit. The document reveals that already in this short period of time, the company has received 42,086 side effects reports, containing 158,893 events, of which 1,223 were deaths.
Table 6 (page 12 in the document), which refers to "Description of Missing Information", presents data on pregnant and lactating women who received the vaccine during the first two and a half months after the vaccine was authorized. According to the data in the table, the company reported 270 cases in .which pregnant women were vaccinated. Of these, "No outcome was provided for 238 pregnancies" That is, the company presented results only for 32 pregnancies.
According to the data in the table, there were 23 miscarriages, two premature births with neonatal death, two miscarriages with intrauterine death, one miscarriage with neonatal death, and one pregnancy with “normal outcome".
This means that out of 32 pregnancies with a known result that the company presented in its report - 28 led to an abortion or a stillbirth, or in other words - to fetal death.
The table states that for five more pregnancies the outcome is "pending", but this figure does not reconcile with the numbers - 32 minus 28 equals four, not five. If these data are correct, it is a huge rate of pregnancy loss - 87.5%, and only one pregnancy result was "normal".
Of the 32 pregnancies with a known result - 28 led to miscarriage or stillbirth, or in other words - fetal death. If these figures are correct, it is a huge rate of pregnancy loss - 87.5%, with only one pregnancy result being "normal".
As mentioned, these data were submitted to the FDA as early as the end of April 2021. Why did the Authority not expose them?
When a new drug or medical device is launched, it is the responsibility of the manufacturer to prove that any subsequent unforeseen event is unrelated to the product. According to the regulations in the US, "it is presumed that all spontaneously reported events are potentially related to the product for the purposes of FDA reporting". However, Pfizer and the FDA ignored reports of adverse events involving fetal death despite the timing, declaring the vaccine as "safe and effective" for pregnant women. Moreover, the authorities strongly recommend that pregnant women be vaccinated, and even put pressure on them to do so, while silencing any discussion on the subject.
The dire results of toxicity studies on fertility and pregnancy in animals were well known to health authorities long before Pfizer was given the emergency use permit, and data on the dire results emerging from Pfizer's report were passed to the FDA as early as late April 2021. So why were all these findings concealed? Even when they were required to reveal them under the Freedom of Information Act, the authorities still tried to keep them hidden, and the FDA even demanded that the documents be kept from the public for decades. Why did regulators not require the company to continue investigating the (significantly) increased rate of animal abortions? Why has the pregnancy and fertility risk category changed from B2 to B1? And why did Pfizer and the FDA ignore what appeared to be a huge rate of reports of miscarriages and stillbirths near time of the vaccination? All of these questions remain unanswered.
Under these conditions can it be argued that there is no research evidence that the vaccine can cause problems in pregnancy or fetal development? It is doubtful that this is the case. Beyond the alarming information, what the documents presented here reveal is the fact that health authorities are willing to hide and manipulate data to protect the interests of drug companies, rather than the public they are supposed to serve, and who do not stop even when it comes to the most vulnerable population, pregnant women and babies. According to Pfizer's own information from both animal studies and clinical trials, at least some cases of miscarriages and stillbirths are related to the experimental vaccines. How many? We will not know until the full data is revealed - a time that could cost the lives of many more babies.
Make a change today!
"Real Time" Magazine is an online, self-funded news magazine, founded with a firm idealogy to make a different, alternative, uncensored quality news, accessible and direct to and with the public.
Our team members currently work as volunteers and we would DEEPLY appreciate and love your support, so we can keep provide what is direly missing in mainstream media.
click for single / monthly support
Thank you ??
תגובות לכתבה זו יועברו לאישור לפני פרסום. אנא השתמשו בשפה מכבדת ואם מצאתם טעות, אנא צרפו נימוק ענייני וקישור למקור הטענה.

Report
Make a change today!
"Real Time" Magazine is an online, self-funded news magazine, founded with a firm idealogy to make a different, alternative, uncensored quality news, accessible and direct to and with the public.
Our team members currently work as volunteers and we would DEEPLY appreciate and love your support, so we can keep provide what is direly missing in mainstream media.
click for single / monthly support
Thank you ??
You might also like...
Hamas October 7 massacre Did Not Happen in A Vaccum: UNRWA has built the infrastructure and fueled the hatred
The exposé according to which UNRWA employees participated in the October 7 massacre shocked the world. But did they all really not know? The evidence showing that UNRWA is systematically and consistently engaged in fueling hatred and was involved up to its neck in terror activities has been presented to world governments and the UN since at least 2003
"I don't 'feel' unsafe at MIT. I AM unsafe. As an Israeli and a Jew, I walk on campus with a target on my back"
MIT, Harvard and Penn presidents are scheduled to testify today before Congress on the increasing antisemitism on campuses. Meanwhile, even after more than 1700 Jewish and Israeli MIT students issued a letter to the administration warning about the tsunami of hatred - the university still refuses to act
Data from Israel's largest HMO reveal: an increase in the number of cancer diagnoses starting in 2021
The data from 'Clalit', obtained through a Freedom of Information request, also indicate an increase in the incidence of breast cancer starting in 2021, coinciding with the start of the COVID-19 vaccination campaign in Israel. The number of diagnoses is 4.5 to 5 times greater than the number among the unvaccinated
Data from Israel's HMOs reveal: a decline in the number of pregnant women in their first trimester starting in February 2021, immediately after the start of the Covid-19 vaccination rollout
New data from Israel's HMOs reveal: a decline in the number of pregnant women in their first trimester starting in February 2021, immediately after the start of the Covid-19 vaccination rollout
"We can’t locate a signed agreement with Pfizer": Did the Netanyahu government and the Israeli MoH mislead the Israeli public and the world? New documents reveal – the deal between Israel and Pfizer was signed before their vaccine even received EUA
"We can’t locate a signed agreement with Pfizer", The Israeli MoH stated in a court hearing two weeks ago. However, last week the ministry suddenly announced that the missing document was found. But this story is only the prelude to the real drama: the new documents reveal that the ministry actively attempted to conceal the fact that the deal with Pfizer, including the agreement for a trial on Israeli citizens, was signed before the vaccine received the emergency permit from the FDA, and created a false representation according to which it was signed later
648 cases of side effects were reported to the VAERS system on breastfed infants whose mothers received the Covid-19 vaccine
Real-Time Magazine analysis reveals a concerning issue almost no one talks about: serious side effects and deaths of breastfed infants whose mothers received the COVID-19 vaccine
Breaking: Leaked Video Reveals Serious Side-Effects Related to the Pfizer COVID-19 Vaccine Covered Up by the Israeli MOH
The Israeli MOH had no adverse events reporting system for the entire year of 2021. They commissioned a research team to analyze the reports from a new system implemented on December 2021. A leaked video reveals that in June, the researchers presented serious findings to the MOH, that indicated long-term effects, including some not listed by Pfizer, and a causal relationship – so the Ministry published a manipulative report, and told the public that no new signal was found
"You violated the community rules": what is behind the increasing censorship policy of Facebook and Google?
A lawsuit recently filed in the US accuses Biden officials of conspiring with Facebook, Twitter and YouTube to censor free speech. The lawsuit comes on the footsteps of an investigation launched by the BMJ into the "Fact Checkers" network operated by Facebook. What stands behind these companies censorship policy? Accumulating information points to enormous economic and political interests
Breaking: The Israeli MOH is hiding a study it conducted, showing a 2-4 times higher rate of adverse events reports following Pfizer COVID vaccine in kids aged 5-11 vs ages 12-17
The study, commissioned by the MOH, also indicates new adverse events not reported in Pfizer's leaflet, and that some adverse events last more than a year. Despite being aware of these findings, the MOH is hiding them from the public and has recently authorized the booster dose for ages 5-11 and is preparing to approve the vaccine for infants
Breaking: 58 babies who received mRNA COVID-19 vaccines suffered life-threatening adverse events
An analysis of VAERS reports shows that contrary to the FDA's briefing document claiming that the majority of adverse events in Pfizers' clinical trial were non-serious – at least 58 cases of life-threatening side effects in infants under 3 years old who received mRNA vaccines were reported. For some, it is unclear if they survived. It is also unclear why the infants were vaccinated, and whether they were part of the clinical trials. However, in the upcoming FDA meeting on Wednesday, the FDA will not be able to argue it did not know












